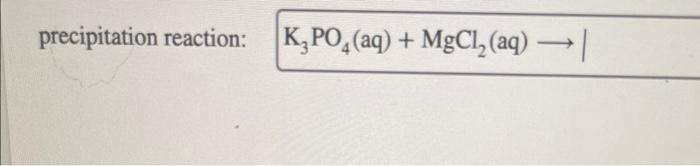K3po4 + mgcl2 precipitation reaction – The precipitation reaction between K3PO4 and MgCl2, a captivating chemical phenomenon, unveils the intricate interplay of ions and the formation of a solid precipitate. This reaction, a cornerstone of chemistry, offers a gateway into understanding the principles governing chemical interactions and their applications in diverse fields.
Delving into the intricacies of this reaction, we will unravel the balanced chemical equation, decipher the stoichiometry, and explore the mechanisms driving the precipitation process. The properties of the precipitate, its applications, and the potential environmental implications will be meticulously examined, providing a comprehensive overview of this fascinating chemical transformation.
Chemical Reaction between K3PO4 and MgCl2: K3po4 + Mgcl2 Precipitation Reaction
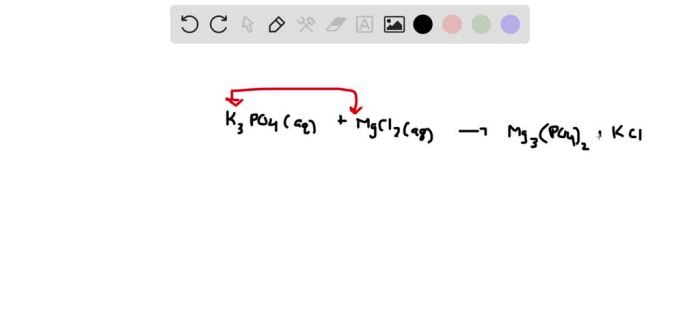
The chemical reaction between potassium phosphate (K3PO4) and magnesium chloride (MgCl2) is a precipitation reaction that results in the formation of magnesium phosphate (Mg3(PO4)2) as a solid precipitate. Precipitation reactions are important in various fields, such as analytical chemistry, environmental science, and industrial processes.
Chemical Equation and Stoichiometry, K3po4 + mgcl2 precipitation reaction
The balanced chemical equation for the reaction is:
2K3PO4 + 3MgCl2 → Mg3(PO4)2 ↓ + 6KCl
The mole ratio between K3PO4 and MgCl2 is 2:3, indicating that for every 2 moles of K3PO4, 3 moles of MgCl2 are required for complete reaction.
Reaction Mechanism
The reaction proceeds via a series of steps involving the exchange of ions between the reactants. Initially, the K+ and Mg2+ ions in solution undergo ion exchange, leading to the formation of Mg3(PO4)2 nuclei. These nuclei then grow by further ion exchange and aggregation, eventually forming a solid precipitate.
Properties of the Precipitate
Magnesium phosphate (Mg3(PO4)2) is an amorphous precipitate that is insoluble in water. It has a white or off-white appearance and a crystalline structure. The precipitate is stable under ambient conditions and is commonly used as a fertilizer in agriculture.
Environmental Implications
The precipitation reaction between K3PO4 and MgCl2 has potential environmental implications. The release of chloride ions (Cl-) into the environment can lead to increased salinity levels in water bodies, which can harm aquatic life. Additionally, the use of K3PO4 as a fertilizer can contribute to eutrophication, a process that leads to excessive algal growth and oxygen depletion in water bodies.
Essential FAQs
What is the significance of the precipitation reaction between K3PO4 and MgCl2?
The precipitation reaction between K3PO4 and MgCl2 is significant because it demonstrates the fundamental principles of precipitation reactions, involving the formation of a solid precipitate from a solution. It also highlights the importance of stoichiometry in chemical reactions, as the mole ratio between the reactants determines the amount of precipitate formed.
What are the applications of the precipitate formed in the reaction between K3PO4 and MgCl2?
The precipitate formed in the reaction between K3PO4 and MgCl2, magnesium phosphate, has applications in various fields, including agriculture as a fertilizer, in medicine as an antacid, and in industry as a flame retardant.
What are the environmental implications of the precipitation reaction between K3PO4 and MgCl2?
The precipitation reaction between K3PO4 and MgCl2 can have potential environmental implications if large amounts of the reactants or the precipitate are released into the environment. The reactants and the precipitate can alter the pH of water bodies and affect aquatic life.