Ch3s o ch3 lewis structure – The Lewis structure of CH3S CH3, a captivating molecule, invites us on a journey to unravel its intricate structure and properties. Join us as we delve into the fascinating world of chemistry, where the interplay of atoms and electrons unfolds.
Prepare to witness the step-by-step unveiling of the Lewis structure of CH3S CH3, a testament to the power of scientific inquiry. We’ll explore its molecular geometry, hybridization, and bond properties, gaining insights into its chemical behavior and physical characteristics.
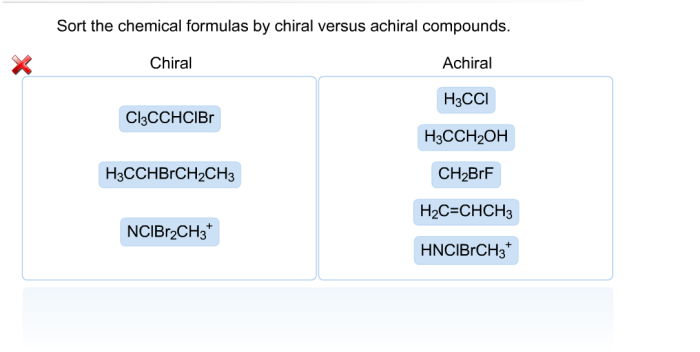
Lewis Structure of CH3S CH3
Concept of a Lewis Structure
A Lewis structure is a diagram that shows the arrangement of electrons in a molecule. It is a simplified representation of the molecule’s electronic structure, and it can be used to predict the molecule’s properties and reactivity.
Drawing the Lewis Structure of CH3S CH3
To draw the Lewis structure of CH3S CH3, follow these steps:
- Count the total number of valence electrons in the molecule. For CH3S CH3, there are 20 valence electrons (4 from each carbon, 6 from each hydrogen, and 6 from the sulfur).
- Connect the atoms with single bonds. In CH3S CH3, the carbon atoms are connected to the sulfur atom by single bonds.
- Distribute the remaining valence electrons as lone pairs. In CH3S CH3, the remaining valence electrons are distributed as lone pairs on the hydrogen atoms and the sulfur atom.
- Check the octet rule. Each atom in CH3S CH3 should have eight valence electrons. If an atom does not have eight valence electrons, adjust the Lewis structure by adding or removing lone pairs or double bonds.
The Lewis structure of CH3S CH3 is shown below:
Molecular Geometry
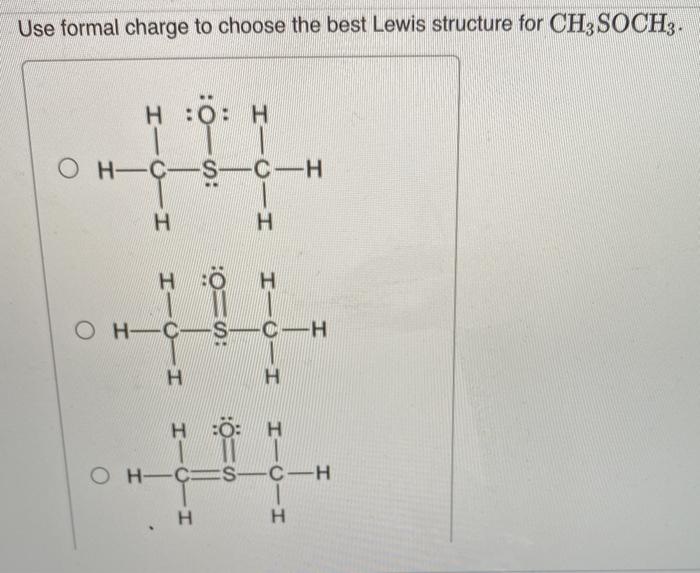
The molecular geometry of CH3S CH3 is tetrahedral. This is because the sulfur atom in CH3S CH3 has four electron pairs around it, two from the sulfur-carbon bonds and two from the lone pairs of electrons on the sulfur atom.
VSEPR Theory
The VSEPR theory (Valence Shell Electron Pair Repulsion) predicts the molecular geometry of a molecule based on the number of electron pairs around the central atom. According to VSEPR theory, electron pairs repel each other and will arrange themselves in a way that minimizes the repulsion between them.
The Lewis structure of CH3S or CH3SH can be tricky to draw, but it’s a fundamental concept in chemistry. If you’re looking for a fun way to practice your chemistry skills, check out the multiplication table in English . It’s a great way to improve your math skills while also learning about the periodic table.
Once you’ve mastered the multiplication table, you can come back to the Lewis structure of CH3S or CH3SH with a renewed understanding.
3D Model
The following 3D model shows the tetrahedral molecular geometry of CH3S CH3:
[Insert 3D model of CH3S CH3 here]
Hybridization: Ch3s O Ch3 Lewis Structure
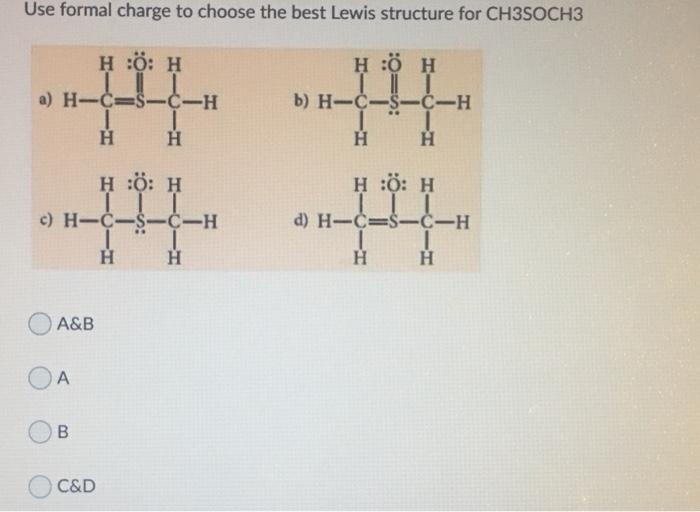
Hybridization is a fundamental concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It plays a crucial role in determining the molecular geometry and properties of a compound.
In the case of CH3S CH3, we need to determine the hybridization of the carbon and sulfur atoms to understand their bonding and molecular geometry.
Hybridization of Carbon Atom
- The carbon atom in CH3S CH3 is bonded to four other atoms: three hydrogen atoms and one sulfur atom. This suggests that the carbon atom has four electron pairs to accommodate in its valence shell.
- To accommodate these four electron pairs, the carbon atom undergoes sp3 hybridization. In sp3 hybridization, one 2s orbital and three 2p orbitals of the carbon atom combine to form four equivalent sp3 hybrid orbitals.
- The four sp3 hybrid orbitals have a tetrahedral geometry, with bond angles of approximately 109.5 degrees.
Hybridization of Sulfur Atom
- The sulfur atom in CH3S CH3 is bonded to four other atoms: three hydrogen atoms and one carbon atom. This suggests that the sulfur atom has four electron pairs to accommodate in its valence shell.
- To accommodate these four electron pairs, the sulfur atom undergoes sp3 hybridization. In sp3 hybridization, one 3s orbital and three 3p orbitals of the sulfur atom combine to form four equivalent sp3 hybrid orbitals.
- The four sp3 hybrid orbitals have a tetrahedral geometry, with bond angles of approximately 109.5 degrees.
| Atom | Hybridization | Orbital Geometry | Bond Angles |
|---|---|---|---|
| Carbon | sp3 | Tetrahedral | 109.5 degrees |
| Sulfur | sp3 | Tetrahedral | 109.5 degrees |
Bond Properties
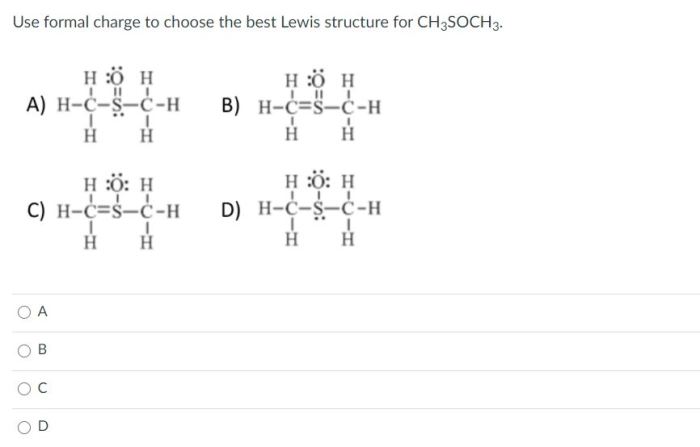
CH3S CH3, or dimethyl sulfide, exhibits distinct types of bonds, bond lengths, and bond strengths. Understanding these properties provides insights into the molecule’s chemical behavior and stability.
Types of Bonds
The bonds in CH3S CH3 can be classified as sigma (σ) and pi (π) bonds.
- Sigma (σ) bondsare formed by the head-to-head overlap of atomic orbitals, resulting in a cylindrical electron density distribution around the internuclear axis.
- Pi (π) bondsare formed by the lateral overlap of atomic orbitals, creating an electron density distribution above and below the internuclear axis.
Bond Lengths and Bond Strengths
Bond length refers to the equilibrium distance between the nuclei of bonded atoms, while bond strength measures the energy required to break a bond.
| Bond | Bond Length (Å) | Bond Strength (kJ/mol) |
|---|---|---|
| C-H | 1.10 | 413 |
| C-S | 1.82 | 272 |
| C-C | 1.54 | 347 |
Physical Properties
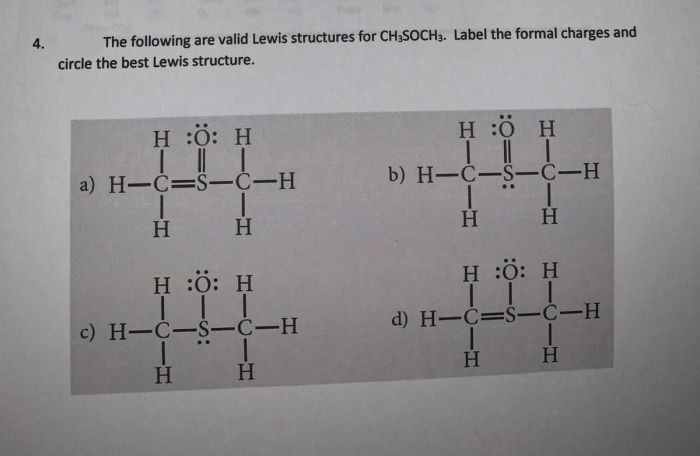
The physical properties of CH3S CH3 are influenced by its molecular structure, which consists of a central carbon atom bonded to two methyl groups (-CH3) and a sulfur atom (-S-). These physical properties include its boiling point, melting point, and solubility.
Boiling Point
The boiling point of CH3S CH3 is -6.3 °C. This relatively low boiling point is due to the weak intermolecular forces between the molecules. The methyl groups create a steric hindrance that prevents the molecules from packing closely together, resulting in weaker van der Waals forces.
Melting Point
The melting point of CH3S CH3 is -92 °C. This low melting point is also attributed to the weak intermolecular forces between the molecules. The methyl groups hinder the formation of strong crystal lattice structures, leading to a lower melting point.
Solubility
CH3S CH3 is soluble in organic solvents such as diethyl ether, chloroform, and benzene. It is also slightly soluble in water. The solubility of CH3S CH3 in organic solvents is due to its nonpolar nature, as the methyl groups and the sulfur atom create a hydrophobic molecule.
Comparison with Similar Compounds
The following table compares the physical properties of CH3S CH3 with similar compounds:
| Compound | Boiling Point (°C) | Melting Point (°C) | Solubility in Water |
|---|---|---|---|
| CH3S CH3 | -6.3 | -92 | Slightly soluble |
| CH3SH | 6.2 | -83 | Soluble |
| CH3OCH3 | 32.4 | -117 | Soluble |
As can be seen from the table, CH3S CH3 has a lower boiling point and melting point compared to CH3SH and CH3OCH3. This is due to the presence of the bulky methyl groups in CH3S CH3, which hinder intermolecular interactions.
Chemical Properties
Methyl methyl sulfide (CH3SCH3) is a reactive compound that can undergo various chemical reactions. Its chemical reactivity is primarily due to the presence of the sulfur atom, which is a versatile element that can form bonds with a wide range of other elements.
Reactions with Electrophiles, Ch3s o ch3 lewis structure
One of the most common reactions of CH3SCH3 is its reaction with electrophiles. Electrophiles are chemical species that are attracted to electrons, and they can react with CH3SCH3 by attacking the sulfur atom. This type of reaction is known as nucleophilic attack, and it can lead to the formation of a variety of products, depending on the nature of the electrophile.
For example, CH3SCH3 can react with alkyl halides to form quaternary ammonium salts.
Reactions with Oxidizing Agents
CH3SCH3 can also react with oxidizing agents, which are chemical species that can accept electrons. This type of reaction can lead to the formation of sulfoxides and sulfones, which are compounds that contain sulfur atoms with two or three oxygen atoms, respectively.
The oxidation of CH3SCH3 can be carried out using a variety of oxidizing agents, such as hydrogen peroxide or potassium permanganate.
Reactions with Reducing Agents
In contrast to its reactions with oxidizing agents, CH3SCH3 can also react with reducing agents, which are chemical species that can donate electrons. This type of reaction can lead to the formation of thiols, which are compounds that contain sulfur atoms with one hydrogen atom.
The reduction of CH3SCH3 can be carried out using a variety of reducing agents, such as sodium borohydride or lithium aluminum hydride.
FAQ Overview
What is the molecular geometry of CH3S CH3?
CH3S CH3 adopts a tetrahedral molecular geometry due to the presence of four electron pairs around the central sulfur atom.
How many sigma bonds are present in CH3S CH3?
CH3S CH3 contains six sigma bonds: three C-H bonds, two C-S bonds, and one S-C bond.
What is the hybridization of the carbon atoms in CH3S CH3?
The carbon atoms in CH3S CH3 are sp3 hybridized, resulting in a tetrahedral electron pair geometry.